phytosqualane
The facts about phytosqualane
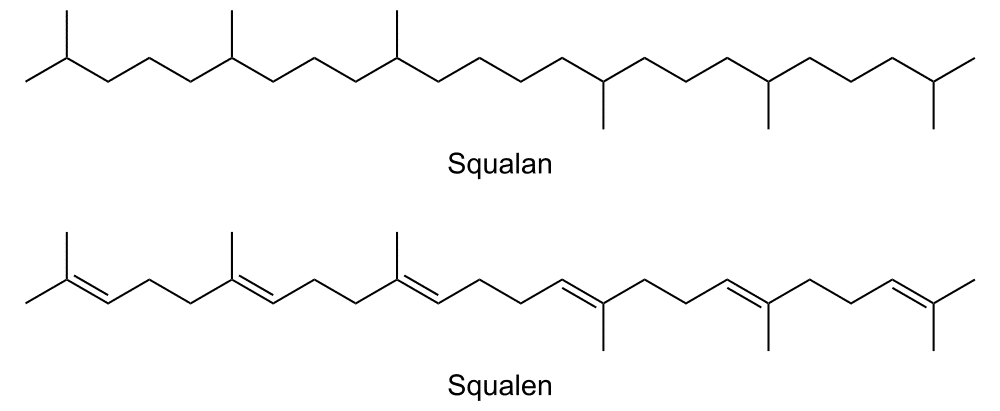
Phytosqualane or squalane (INCI: Squalane; IUPAC name: 2,6,10,15,19,23-hexamethyltetracosane; CAS 111-01-3) is highly valued as an ingredient in cosmetics. In its pure state, it is a liquid, colorless, odorless, and tasteless hydrocarbon oil with good physical and chemical stability. Its properties are reflected in its high boiling point of 210-215 °C (at 1 torr pressure) and considerable resistance to chemical oxidation, which eliminates the need for preservatives. Squalane is present in small quantities in the lipid layers of the skin; together with its precursor squalene, it prevents moisture loss and contributes to skin suppleness and elasticity. With its sensory profile, biocompatibility with the skin, chemical and physical stability and moisturizing effect, it has become a favorite ingredient of cosmetic developers. Looking at its technical properties, it impresses with its good emulsifiability, excellent dispersibility and compatibility with other ingredients. It is soluble in all common cosmetic bases and can be used in all types of formulations without restrictions. After removal of impurities, which may be present in varying amounts depending on the source and type, it is completely non-toxic and non-irritating.
Conventional sources of squalane or phytosqualane
Conventionally, squalane is obtained by catalytic hydrogenation of squalene (6E,10E,14E,18E-2,6,10,15,19,23- hexamethyltetracosa-2,6,10,14,18,22-hexaene). Squalene is a natural triterpene hydrocarbon and one of the most important lipids of human skin cells. It is formed in the sebaceous glands; its contribution to total lipids here is up to 13%. Its concentration varies from skin area to skin area; the amount secreted also differs from person to person, ranging from 125 mg to 475 mg per day. Squalene is also found in plants, prokaryotes, yeast, and microalgae.
For commercial use, squalene has traditionally been extracted from the cod liver oil of the deep-sea shark. Fish liver oil has a long history as a health food in China, Japan, and Korea. Shark liver oil was mentioned as early as the mid-16th century in a Chinese manual on traditional remedies. In the early 20th century, Marcelet, Chapman and Tsujimoto independently succeeded in isolating a polyunsaturated hydrocarbon from shark liver oil. Tsujimoto, a chemist in the field of oils and fats at the Industrial Research Institute in Tokyo, correctly decoded the formula as C30H50 and named it squalene, in reference to the fact that sharks belong to the Squalidae family. In 1931, the 1937 Nobel Prize winner Paul Karrer at the University of Zurich, Switzerland, published the complete chemical synthesis, providing unequivocal proof of the chemical structure. Due to its numerous double bonds, squalene was initially considered insufficiently stable for widespread practical use, until Sebastien Sabetay of the French company Laserson & Sabetay came up with the idea around 1950 of hydrogenating it to squalane, or perhydrosqualene, as it was then called. This enabled the commercial use of the substance as an important cosmetic ingredient.
At the end of the 1970s, the Japanese company Kuraray launched the first fully synthetically produced squalane on the market. It was the product of the base-catalyzed reaction of 1,3-butadiyne, a byproduct of acetylene synthesis from natural gas, and two molecules of geranylacetone-a molecule produced by the ton for the synthesis of isophytol, an intermediate in the production of vitamins E and K1, and other terpenoids-with subsequent hydrogenation of the resulting adduct. Despite its high degree of purity, the cost of the raw material, due to the multi-step manufacturing process, prevented its use in cosmetics on a large scale.
Phytosqualane from olive oil
Squalene is found in smaller amounts in many vegetable oils, for example, olive oil containing 0.2-0.5%. In fact, before more precise analytical methods such as gas or liquid chromatography were possible, squalene was routinely added to some olive oils to increase their value, as quality was determined by squalene content. Squalene is also a component of palm oil; its content in palm fatty acid distillate (PFAD) is up to 0.8%, and 0.06-0.1% in unprocessed oil. Amaranth seed oil contains 6-8% squalene; other vegetable oils also contain squalene.
Although the squalene content of olive oil is usually less than 0.5%; concentrations of 60-75% may be present in the unsaponifiables of the oil. However, direct extraction appeared uneconomical until the 1980s. Then several Spanish companies patented their processes for extracting and refining squalene from the residues produced during oil extraction from olives. Eventually, Hispano Quimica S.A. – which was acquired by Cognis in 2000 and BASF in 2010 – was able to market “phytosqualane” from olive oil deodorizer distillates (OODD). The concentrated waste product produced in the final step of olive oil refining contains up to 30% squalene. Improvements to this technology were eventually patented.
Risks with squalane
Considering the many sources from which the raw material squalene comes, even 100% pure squalane contains some impurities. Squalene from shark may contain environmental pollutants, such as polychlorinated biphenyls (PCBs), dioxins, and heavy metals, which accumulate as bioconcentrates in the liver. In addition, squalane contains impurities, which accumulate during biosynthesis, such as low-volatile triacylglycerols and glyceryl ethers and up to 0.1% C19 hydrocarbon pristane with high irritation potential. Squalene from olive oil extraction waste may be contaminated with processing by-products, such as plant waxes, free fatty acids, phytosterols, and neutralization by-products. Due to such contaminants, purification of the raw material squalene and/or squalane end products is enormously important to ensure safe use in cosmetics.
As with other natural raw materials, the availability of squalane is associated with vagaries of weather and harvest. In addition, squalane has been caught in the crossfire of criticism with campaigns to protect the endangered shark used to produce squalane. A campaign by Oceana in 2005 was ultimately instrumental in getting several cosmetics manufacturers to publicly commit to stopping or phasing out the use of shark squalane in their products.
A campaign by the Environmental Justice Foundation at the end of 2013 targeted the “black market” for shark squalane, which was offered as or mixed with squalane extracted from olives – even though it is possible to distinguish between the two by means of analytical tests and the listing of ingredients on the products must indicate shark squalane.
Biotechnological approaches to the extraction of phytosqualane
Only recently have advances in biotechnology offered solutions to these problems, making it possible to produce pure, stable products on a large scale at low cost and from renewable sources. While it was suspected as early as the 1920s that squalene plays a role in the isoprene pathway that eventually leads to steroids in the human body, evidence for this was not provided until 1964 by Konrad Bloch. He discovered the biosynthesis of cholesterol and was awarded the Nobel Prize for his work.
A new approach based on the isoprenoid pathway deals with the commercial production of phytosqualane from fermentable sugars. b-Farnesene, the natural precursor of squalene, is produced on an industrial scale by fermentation using the common, non-pathogenic yeast Saccharomyces cerevisiae. The yeast is completely removed, followed by a simple chemical binding process similar to natural processes. This in turn eliminates the need to isolate lipophilic and oxidatively unstable squalene from the fermentation biomass. Known hydrogenation and purification technologies can then be used to obtain phytosqualane of high purity. Fig. 1 outlines the process
Flowchart of the process to obtain phytosqualane from sugar:

This manufacturing process offers several advantages. It is a renewable, bio-based process. It is certified by the U.S. Department of Agruculture (USDA) as 100% biobased and recognized by Ecocert. It is robust and reproducible and, together with the availability of the feedstock, ensures the reliable and sustainable production of phytosqualane. The strictly controlled manufacturing process guarantees batch consistency in terms of both chemical and sensory properties, such as odor and/or color.
Since the starting material used in the chemical process, β-farnesene, is a hydrocarbon of high purity, unlike natural squalene, which may contain impurities, the products and by-products of this process are also pure hydrocarbons.
| Substance | Content in % | Remarks |
|---|---|---|
| Phytosqualan | 92-94 | |
| Isosqualan | 3-5 | |
| Monocyclosqualan | 1-3 | |
| Hemisqualane (C15) | 0-1 | |
| Sesquisqualan (C45) | 0-1 |
Phytosqualane – sustainable extraction from sugar cane
The history of squalane or phytosqualane production and its development and commercialization as a cosmetic ingredient has been dominated by the vagaries of the availability and quality of traditional natural sources, the cost of chemical processing, and the shortcomings of earlier fermentation-based techniques. Thanks to modern biotechnology, the enzyme-catalyzed chemical reactions found in nature could be replicated and coupled with traditional chemical processes to create a high-quality source of renewable phytosqualane. The process is based on b-farnesene, a fermentation product of the yeast Saccharomyces cerevisae, and uses a chemical reaction to reproducibly produce phytosqualane that is odorless, stable, and available in a batch-consistent composition of >99% C30 hydrocarbon molecules. The resulting sugar phytosqualane offers high quality and is comparable in performance to shark-derived squalane, but unlike the latter, is derived from a renewable source. Because of this advanced manufacturing process, renewable phytosqualane can be used in cosmetic formulations without the problems of lack of availability or wide variations in quality.
Literature:
- S-K Kim and F Karadeniz,Biological importance and applications of squalene and squalane, in Advances in Food and Nutrition Research, vol 65, S-K Kim, ed, Elsevier, Amsterdam, ch14 (2012) pp 223-233
- Final report on the safety assessment of squalaneand squalene, Int J Toxicol1 37-56 (1982)
- KR Smith and DM Thiboutot,Thematic review series: Skin lipids. Sebaceous gland lipids: Friend or foe? J Lipid Res 49 271-281 (2008)
- RS Greene, DT Downing, PE Pochi and JS Strauss, Anatomical variation in the amount and composition of human skin surface lipid, J Invest Dermatol54 240-247 (1970)
- T Nikkari, PH Schreibman and EH Ahrens, Jr, In vivo studies of sterol and squalene secretion by human skin, J Lipid Res15 563-573 (1974)
- F Bouvier, A Rahier and B Camara,Biogenesis, molecular regulation and function of plant isoprenoids, Prog Lipid Res 44 357-429 (2005)
- M Spanova and G Daum,Squalene—Biochemistry, molecular biology, process biotechnology and applications, Eur J Lipid Sci Technol113 1299-1320 (2011), and references therein
- E Andre and H Canal, Contribition a l’étude des huiles d’animaux marins. Recherches sur le squaléne et la spinacéne, Ann ChimAppl7 69-112 (1927)
- M Tsujimoto, An unsaturated hydrocarbon in shark liver oil, J Chem Ind Jpn19 277-281 (1916)
- P Karrer and A Helfenstein, Synthesis of squalene,HelvChim Acta14 78-85 (1931)
- S Sabetay, Five years of perhydrosqualene—A revolution in cosmetics, RiechstoffArom5 274-276 (1955)
- T Nishida, Y Ninagawa, K Itoi and Y Fujita, New industrial synthesis of squalane, Bull Chem Soc Jpn56 2805-2810 (1983)
- J Grossfeld and H Timm, A new characteristic for oliveoil, Z UntersLebensm77 249-253 (1939)
- MT Gapor and AR Hazrina, Squalene in oils and fats, Palm Oil Develop32 36-40 (2000)
- CA Auguet, A new source of Ssqualane, Drug Cosmet Ind82 51-53 (1988)
- W Dickhart, The squalene contents of various oils,Am J Pharm127 359-361 (1955)
- K Taufel, H Heinisch and W Heimann, The distribution of squalenes in vegetable fats,Biochem Z303 324-8 (1940)
- MM Storelli, E Ceci, A Storelli and GO Marcotrigiano, Polychlorinated biphenyl, heavy metal and methylmercury residues in hammerhead sharks: Contaminant status and assessment, Mar Pollut Bull 46 1035-1039 (2003)
- A Gasparoli, C Mariani and MG Fedrigucci, Squalane: Differentiation between vegetable and animal origin, Riv Ital Sostan Grasse73 293-302 (1996)
- Gasparoli, CMariani, ME Gaboardi, G Morchio and G Santus, About detection of animal squalene/squalene in vegetable products used in the cosmetic field, Riv Ital Sostan Grasse89 4-28 (2012)
- The isoprene rule and the biogenesis of terpenic compounds. Experientia9 357-67 (1953)
- F Mantzouridou and MZ Tsimidou, Observations on squalene accumulation in Saccharomyces cerevisiaedue to the manipulation of HMG2 and ERG6, FEMS Yeast Res 10 699-707 (2010), and references therein
- S.Chandran, JT Kealey and CDReeves, Microbial production of isoprenoids,Process Biochem 46 1703-1710 (2011)
- L Zhao, W Chang, Y Xiao, HLiu and P Liu, Methylerythritol phosphate pathway of isoprenoid biosynthesis, Ann Rev Biochem82 497-530 (2013)
- Effectiveness and tolerability of a squalaneand dimethicone-based treatment for head lice. Martínez de Murguía Fernández L, Puig Algora G, Bajona Roig M, Bacchini G.Parasitol Res. 2021 May;120(5):1883-1890