Malates
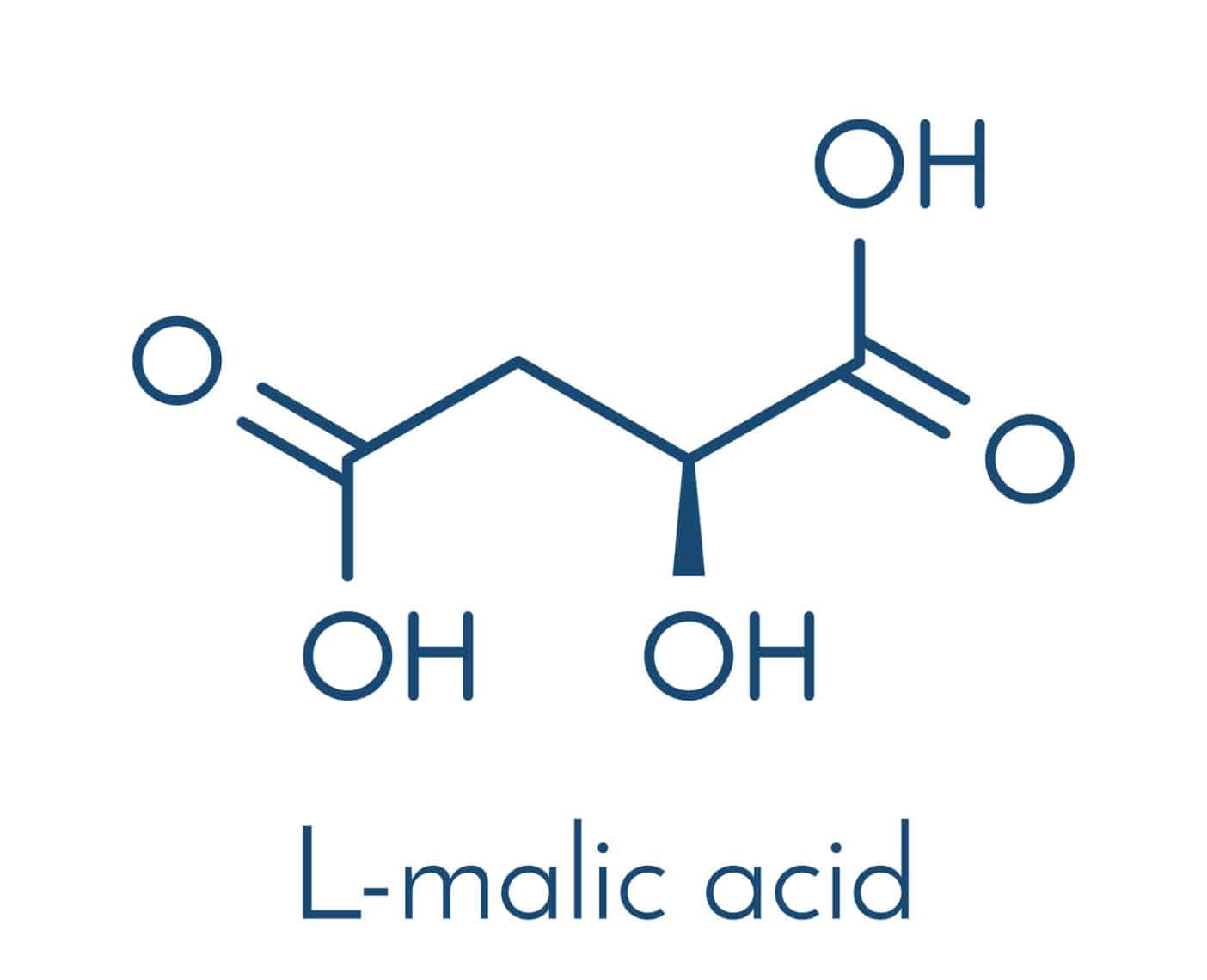
Malates comes from reaction of malic acid. Malic acid (also known as 2-hydroxy succinic acid) is found in the metabolism of all cells and is involved in many metabolic processes in the human organism. In nature, it is mainly found in the unripe fruit of numerous fruit varieties.
And not only in apples themselves, but also in quinces, gooseberries, grapes and some other varieties. Among other things, it is responsible for the sour flavour of unripe fruit. As the fruit ripens, the malic acid content decreases and the sugar content increases. In this way, nature prevents animals from eating the unripe fruit and spreading the unripe seeds, which would be detrimental to the reproduction of the tree or shrub.
The acid was first isolated from apple juice in 1785 by the German-Swedish physicist and pharmacist Carl Wilhelm Scheele.
The salts and esters of malic acid are called malates. And these are at least as interesting. For example, potassium malate (E351), sodium malate (E350) and calcium malate (E352) are listed as official food additives in the food industry. They are used as stabilisers, acidity regulators and humectants. They are mainly used in fruit juices, jams and jellies as well as tinned fruit and vegetables.
Both malic acid and malates are considered to be completely harmless to health.
Malates and their benefits
Calcium malate contains a high proportion of elemental calcium and thus contributes to the maintenance of teeth and bones, an optimal energy metabolism and normal muscle function.
Potassium malate, the double potassium salt of malic acid, has a flavour-enhancing effect and can help to reduce the use of sweeteners in sugar-free foods. Even more interesting, however, is the fact that potassium malate supports the effect of antioxidants.
Magnesium malate is increasingly being used as a food supplement. In corresponding products, the magnesium is bound to the salt of malic acid. In principle, magnesium malate is absorbed and metabolised by the body much faster than magnesium citrate or magnesium oxide, for example, because the human organism can break down malates more easily. This excellent bioavailability primarily benefits our muscles, but also our nerves and our psyche. Overall, the substance ensures smooth interaction between nerves and muscles. It improves the performance of our cells and contributes to improved stimulus transmission.
Zinc malate, the zinc salt of malic acid, protects against oxidative stress and supports the immune system. It plays an important role in maintaining the health of our skin, hair and nails. This special malate is also known as apple zinc.
Malates in cosmetics
Malates have long played an important role in the food industry and are also becoming increasingly interesting as food supplements. The realisation that malates are also of great importance for cosmetics is still relatively new. The fact that they protect against oxidative stress makes them particularly interesting for high-quality skin care products. Malates are also beneficial for sophisticated hair and nail care products, as they have a strengthening and health-preserving effect. They also support other antioxidants in their effect. According to current knowledge, malates are not considered allergenic and an overdose is virtually impossible. They are therefore an ideal solution even for sensitive skin.
If you would like us to develop ready-made cosmetic products with malates for you, please contact us.
Calcium malate profile
INCI: Calcium malate
Alternative names: Calcium-alpha-hydroxysuccinate, Calcium-DL-malate
CAS number: 17482-42-7
EINECS-No.: 241-498-1
Description: colourless solid
Solubility: insoluble in ethanol, slightly soluble in water
Profile potassium malate
INCI: Dipotassium malate
CAS number: 585-09-1
EINECS-No: 209-549-2
State: solid
Field of application: mainly as food additive (E 351) without maximum quantity restriction
Profile magnesium malate
INCI: Magnesium malate
CAS number: 869-06-7
EINECS-No.: 212-784-3
Description: white solid
We like to work with these malates in cosmetics:
| Tradename | INCI | Supplier | Remarks |
|---|---|---|---|
| Solbrol FA | Aqua (and) Lactic Acid (and) Malic Acid (and) Propanediol (and) Citric Acid (and) Glycolic Acid (and) Ascorbic Acid (and) Tartaric Acid (and) Gluconic Acid | Lanxess | |
| IRAFruit A.H.A. | Propylene Glycol (and) Lactic Acid (and) Aqua (and) Solanum Lycopersicum (Tomato) Fruit/Leaf/Stem Extract (and) Citrus Limon (Lemon) Fruit Extract (and) Citrus Grandis (Grapefruit) Fruit Extract (and) Vaccinium Myrtillus Fruit Extract (and) Citric Acid (and) Malic Acid | Rahn AG | |
| DISM10M5 | Diisostearyl Malate (and) Silica | Kobo | |
| PELEMOL DISM | Diisostearyl Malate | Phoenix | |
| Ceraphyl 45 | Diethylhexyl Malate | Ashland | |
| Schercemo DISM Ester | Diisostearyl Malate | Lubrizol | |
| PELEMOL DISM-V | Diisostearyl Malate | Phoenix | |
| COSMACOL EMI | Di-C12-13 Alkyl Malate | Sasol | |
| PELEMOL D5R-V | Propanediol Dicaprylate/Caprate (and) Diisostearyl Malate | Phoenix | |
| Ama-oil | Helianthus Annuus (Sunflower) Seed Oil (and) Amaranthus Caudatus Seed Extract (and) Diisostearyl Malate (and) Rosmarinus Officinalis (Rosemary) Leaf Extract | Provital Group |
Malates in cosmetics
Malates have long played an important role in the food industry and are also becoming increasingly interesting as food supplements. The realisation that malates are also of great importance for cosmetics is still relatively new. The fact that they protect against oxidative stress makes them particularly interesting for high-quality skin care products. Malates are also beneficial for sophisticated hair and nail care products, as they have a strengthening and health-preserving effect. They also support other antioxidants in their effect. According to current knowledge, malates are not considered allergenic and an overdose is virtually impossible. They are therefore an ideal solution even for sensitive skin.
Literature:
Dual Effects of Alpha-Hydroxy Acids on the Skin.
Tang SC, Yang JH.Molecules. 2018 Apr 10;23(4):863
Hsiao YP, Lai WW, Wu SB, Tsai CH, Tang SC, Chung JG, Yang JH.Toxins (Basel). 2015 Jan 9;7(1):81-96